A group of scientists from Lawrence Livermore National Laboratory and the Carnegie Institution for Science have managed to discover two completely new forms of gold's molecular structure while studying the precious metal's behaviour under harsh conditions.
Using a powerful laser, scientists rapidly heated a chunk of gold to extreme temperatures, while subjecting it to rapid compression with pressure levels reaching 322 gigapascals - similar to the ones at the Earth's core. The researchers made stunning discoveries when they monitored the changes in the metal's structure using x-rays.
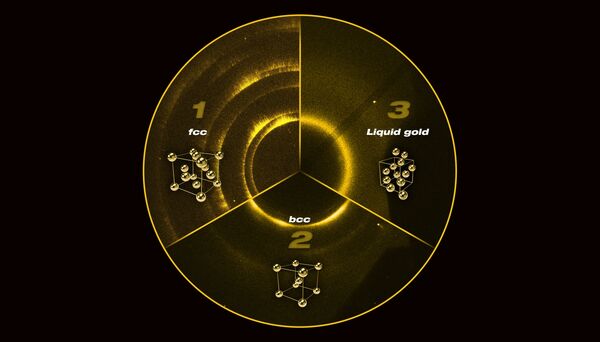
It turned out that when gold is exposed to rapid compression and heating up, it behaves differently, changing its face-centred cubic (fcc) structure, where atoms were located at the "faces" of the molecular cube, to body-centred cubic (bcc) structure, where atoms were pressured to the centre of the cube, at around 220 gigapascals. When the pressure levels reached closer to 330 gigapascals, found at the Earth's centre, the gold turned into liquid.
These two new forms contradict the common belief that gold maintains its structure under harsh pressure, which found its use in various pressure experiments that used gold as a standard. The experiment's results showed that the metal maintains stability in its molecular structure only under gradual pressure rise and under normal temperature.


