How a Rogue Judge May Upend the FDA by Banning the Abortion Pill Mifepristone
23:40 GMT 13.02.2023 (Updated: 21:06 GMT 14.02.2023)
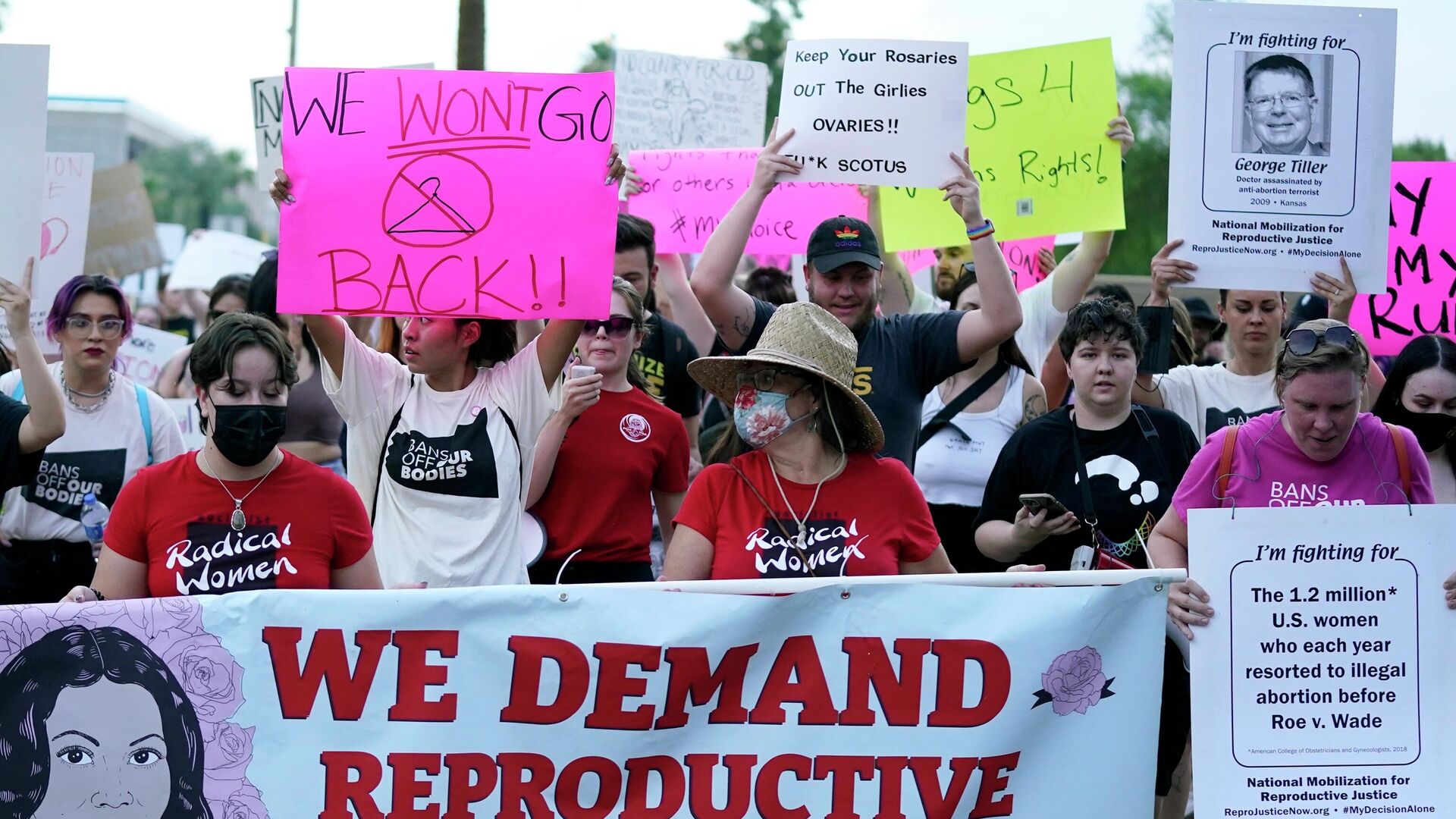
© AP Photo / Ross D. Franklin
Subscribe
More than 20 million Americans had the constitutional right to govern their own bodies stripped away in June, when Roe v. Wade, a nearly 50-year age decision, was overturned by a conservative-majority US Supreme Court. The decision ultimately confirmed an earlier leak of a draft decision by SCOTUS to overturn the landmark 1973 ruling.
Now, another conservative judge is ignoring 20 years of safety data and is likely to further the attack on Americans’ health care rights as an anti-abortion group looks to ban access to mifepristone, a progesterone-inhibiting drug invented in France in the 1980s that is used in medical abortion procedures.
Should mifepristone be banned, doctors may be able to turn to its counterpart misoprostol, which is taken after mifepristone to ensure a medication abortion. More than half of unwanted pregnancies are treated in this manner.
Christina Cauterucci for Slate reports that when taken in higher doses, misoprostol may be able to induce an abortion on its own. The drug was found to be able to do so in Brazil in the 1980s. And because it was originally approved to prevent and treat stomach ulcers, it's possible it may not be subject to a politically-motivated ban.
Trump-Appointed Judge Takes Center Stage
Matthew Kacsmaryk was appointed to serve as a federal judge in Texas by former President Donald Trump in 2019. His confirmation included various bumps, including one instance in which Sen. Chuck Schumer (D-NY) described Kacsmaryk as “narrow-minded” and “bigoted."
“Mr. Kacsmaryk has demonstrated a hostility to the LGBTQ bordering on paranoia,” said Schumer, who was the then-minority leader of the congressional chamber.
“It’s unbelievable that this man has been nominated, and he’s not alone. The parade of narrow-minded, often bigoted people who we’re putting on the bench... One Republican senator rightfully voiced concerns about this man’s fitness. Where are the others?”
US Sen. Susan Collins (R-ME), who was the only Republican senator to vote against Kacsmaryk’s appointment, also pointed out his “extreme statements” on reproductive rights and the LGBTQ community and predicted his “inability to respect precedent and to apply the law fairly and impartially.”
Kacsmaryk, who has described transgender people as suffering from a “mental disorder” and called homosexuality “disordered,” and also claimed “sexual revolutionaries” were responsible for making “the erotic desires of liberated adults” take precedence over fetuses and marriage, was confirmed 52 to 46 in June 2019.
That same judge now holds the future of the abortion pill, mifepristone, in his hands. And thus far, Kacsmaryk has ruled conservative in most cases brought before him, including the protection of Trump’s “Remain in Mexico” immigration program, blocking policies written to protect LGBTQ people from discrimination in the workplace and doctors’ offices, and making it so people under the age of 18 must request parental consent if they wish to obtain free contraception.
Alliance for Hippocratic Medicine v. FDA
Just days after persons across the country voted in favor of protecting abortion rights in the US midterms, a conservative anti-abortion group called Alliance for Hippocratic Medicine filed a lawsuit against the US Food and Drug Administration (FDA), targeting the agency’s 20-year-old approval of mifepristone. The goal, of course, is to eliminate a person’s right to abortion care no matter the state they live in - even if a US citizen lives in a state where abortion is legal.
Alliance for Hippocratic Medicine was incorporated into Texas three months before filing the suit, and claimed residency in Amarillo, thus allowing their lawsuit to fall into Kacsmaryk’s hands after tapping the process of "judge shopping" in order to bring their case before a favorable judge.
“If FDA approval of mifepristone is revoked, 64.5 million women of reproductive age in the US would lose access to medication abortion care, an exponential increase in harm overnight,” said NARAL, a pro-choice nonprofit group.
The lawsuit is calling for the FDA to “withdraw mifepristone and misoprostol as FDA-approved chemical abortion drugs and to withdraw defendants’ actions to deregulate these chemical abortion drugs” in order to “protect women and girls.”
In defense of their case, the FDA cited a study of more than 30,000 American women who underwent abortion care before nine weeks of gestation and found that the efficacy of the pill was 99.6%.
The FDA has responded by asking the judge to deny the motion for a preliminary injunction, and has shot back writing “[the decision] would upend the status quo and the reliance interests of patients and doctors who depend on mifepristone, as well as businesses involved with mifepristone distribution.”
The FDA also warned that any ruling against it would upend the pharmaceutical-drug infrastructure in its entirety.
“More generally, if longstanding FDA drug approvals were so easily enjoined, even decades after being issued, pharmaceutical companies would be unable to confidently rely on FDA approval decisions to develop the pharmaceutical-drug infrastructure that Americans depend on to treat a variety of health conditions,” the FDA wrote.
“A preliminary injunction would interfere with Congress’s decision to entrust FDA with responsibility to ensure the safety and efficacy of drugs. In discharging this role, FDA applies its technical expertise to make complex scientific determinations about drugs’ safety and efficacy, and these determinations are entitled to substantial deference.”
The briefing deadline for the suit is on February 24. Kacsmaryk’s decision is expected any time after that.

