Brought to you by the National Research Nuclear University MEPhI
Carbon is one of the most common chemical elements on Earth. It is part of all organic and many inorganic compounds. Before the end of the 20th century only two of its allotropic forms, diamond and graphite, were known. To date, scientists have discovered plenty of other forms that are already used in electronics, pharmacology and energy.
Chemically modified fullerenes became the next stage in the development of fullerene technology. Substituting doping, which includes substituting one or several carbon atoms with atoms of a different element, is a common modification method. The fullerene’s overall structure remains the same but its electronic composition and chemical activity change.
READ MORE: 'Extremophile' Antarctic Bacteria Could Unlock Secret to Alien Life (VIDEO)
Therefore, substituting doping increases the variability of fullerenes’ characteristics and hence expands the scope of their application.
Carbon’s closest elements in the Periodic Table are usually used as substitutes. They are boron or nitrogen, which have an atomic mass and a size close to that of carbon. Boron- and nitrogen-doped fullerenes are good adsorbents of medical substances and nerve agents. They also successfully adsorb additives.
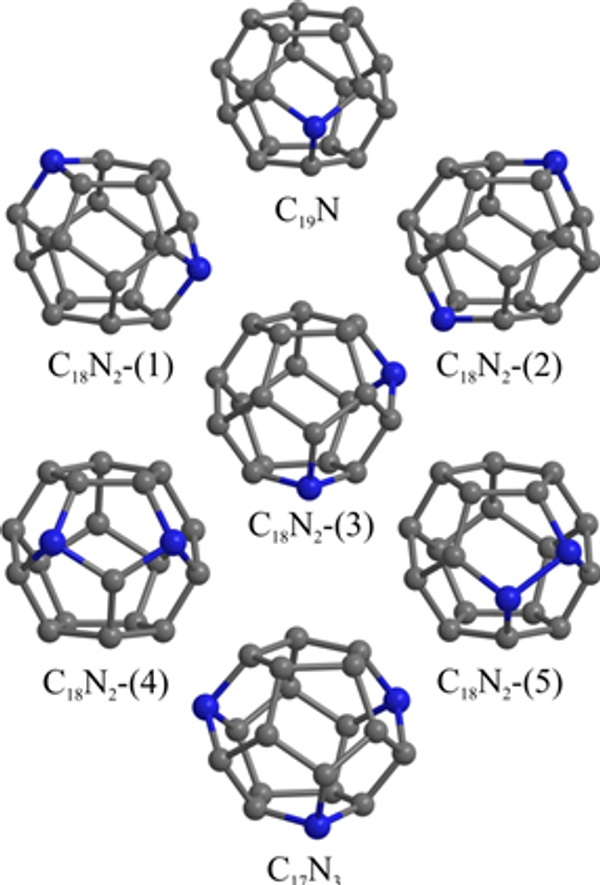
However, a surging interest in their professional synthesis allowed scientists to also discover that production of nitrogen-doped fullerenes have a high share of defective isomers that differ from the others in structure and characteristics. The high temperatures required for synthesis caused the so-called Stone-Wales defect that destabilized fullerenes’ cages. It is important to note that boron-doped fullerenes were heat-resistant.
Interaction of fullerene atoms and distribution of electrons within its cage were described using special mathematical models based on the laws of quantum mechanics. The physicists used both specialized software packages and their own original programs. The most complicated task was to establish the geometry of the saddle point, a fullerene’s configuration when normal thermal excitation becomes irreversible and by all means leads to the defect.
READ MORE: Nanorevolution: How Quantum Dots Created a Medical Breakthrough
MEPhI’s results provided a complete explanation of the stability of doped fullerenes. Based on quantum mechanics equations, the researchers proved that, unlike boron, even one atom of nitrogen can destabilize a fullerene cage because of the nitrogen’s atom having one additional electron.
“We found that it takes 4.93 eV to destroy the original С20 fullerene while it takes only 2.98 eV to destroy a C19N doped fullerene. Clusters with more nitrogen are even less stable. Based on these data, we can conclude that nitrogen-doped fullerenes are highly susceptible to temperature. Lowering the temperature in a reactor by only ~20°C will significantly reduce the share of defective fullerenes,” Konstantin Katin explained.
The publication provoked great international interest among scientists researching production and application of doped fullerenes. Within the next few years, a technology may be developed for synthesizing nitrogen-doped fullerenes at lower temperatures. The technology could solve the problem of defective isomers and ensure that the characteristics of the resulting cluster can be reproduced.