Advertisers in India are trying hard to link their products directly or indirectly to COVID-19 to make them more relevant and attract people's attention during the pandemic.
In the process, various unverified products are being marketed as “coronavirus resistant.” While some advertisements are deploying deceptive images, others are using witty wordplay to fool customers.
Firms accused of building up such false narratives to convince customers to buy their products can be held liable under the Indian Penal Code's Drugs and Magic Remedies (Objectionable Advertisements) Act.
Coronavirus Resistant Mattress
A huge controversy erupted around an advertisement about a 'coronavirus-resistant' mattress placed in a regional daily in March.
Mattress manufacturer 'Arihant' faced backlash for using a false claim to mislead and influence customers. The phrase read, “Anti coronavirus mattress pe soyega India toh badhega India (India will progress if India sleeps on this anti-coronavirus mattress)”.
Tremendous creativity 🙄 pic.twitter.com/SAZdwAUUwm
— Partho Dasgupta (@parthodasgupta) March 13, 2020
The picture of the advert, which priced the mattress at $198 (INR 15,000), went viral on social media. Soon, the mattress manufacturer was booked for violating the law under various acts, including the Disaster Management Act.
The Advertising Standards Council of India (ASCI) has also reached out to the advertiser, which has since agreed to withdraw its claims.
Antimicrobial Coating Solution
In a similar advertisement, a firm named Healife Essentials has been advertising non-toxic, eco-friendly antimicrobial solutions to help safeguard against coronavirus. The firm claims that its product is tested and approved by the Indian Institute of Toxicology Research and Council of Scientific and Industrial Research (CSIR).
Explaining to Sputnik how the solution works, firm partner Kapil Sinh said, "Once there is bacterial or viral infection, this product is positively charged and all the bacteria and viruses are negatively charged. So it attracts them; punctures them and electrocutes them."
Since the advertisement markets the product as a 'safeguard against coronavirus' during the pandemic, the firm partner was asked if the product works against COVID-19 specifically and whether it has been backed by labs or authorities.
"There is no lab in the world which has done it specifically for COVID-19. COVID-19 is one of the coronaviruses. It is good for all of them. Anything which has kilobase pairs above five, it will kill that," Kapil claimed.
Ayurvedic Corona Kit
India's renowned Ayurveda firm Patanjali Ayurved has claimed to find a cure for coronavirus - a pill named 'Coronil and Swasari', made up of Ayurvedic herbs, that the firm claims can cure a person in 5-14 days. The company said that the claims are based on results derived from controlled trials conducted according to scientifically-approved methods.
However, the pill has been embroiled in controversy after India’s ministry dealing with alternative medicines pulled up the firm for not making it aware of the facts of the "claim and details of the stated scientific study". It has asked Patanjali to stop advertising Coronil and Swasari as a COVID-19 treatment until the ministry examines the medicine.
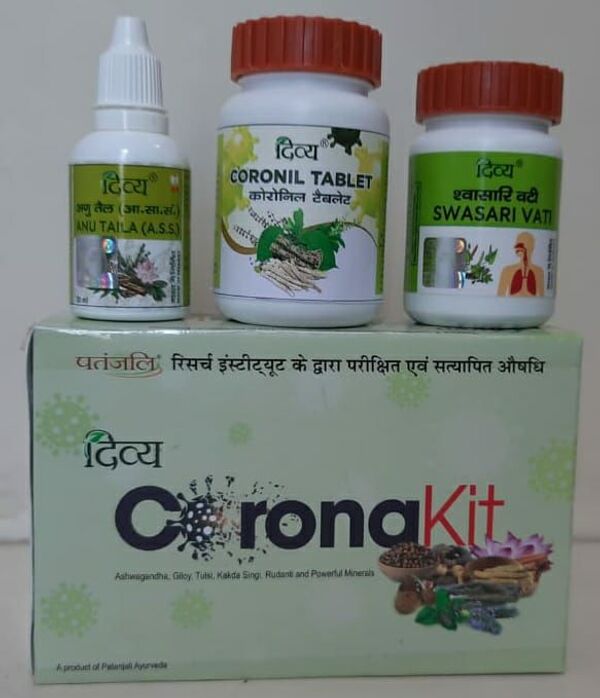
The government of Uttarakhand, the state in which the firm’s manufacturing hub is located, has alleged that the Ayurveda firm was given license to manufacture an "immunity booster" and its application did not mention it as a cure for COVID-19.
Falling in line with the ministry's orders, the firm, founded by well-known yoga instructor Baba Ramdev, has submitted a report detailing the composition and other details about the medicine. The firm continues to stand by its claims and said they would be validated by the results.
Curbing False Narratives
Meanwhile, in order to curb the spread of false claims and information, American e-commerce giant Amazon has de-listed myriad products posing as cures for novel coronavirus and making 'unapproved medical marketing claims'. Similarly, Facebook followed suit by banning all such misleading advertisements.


