https://sputnikglobe.com/20221227/india-conducts-nationwide-inspection-of-drug-production-facilities-in-wake-of-gambia-child-deaths-1105825122.html
India Conducts Nationwide Inspection of Drug Production Facilities in Wake of Gambia Child Deaths
India Conducts Nationwide Inspection of Drug Production Facilities in Wake of Gambia Child Deaths
Sputnik International
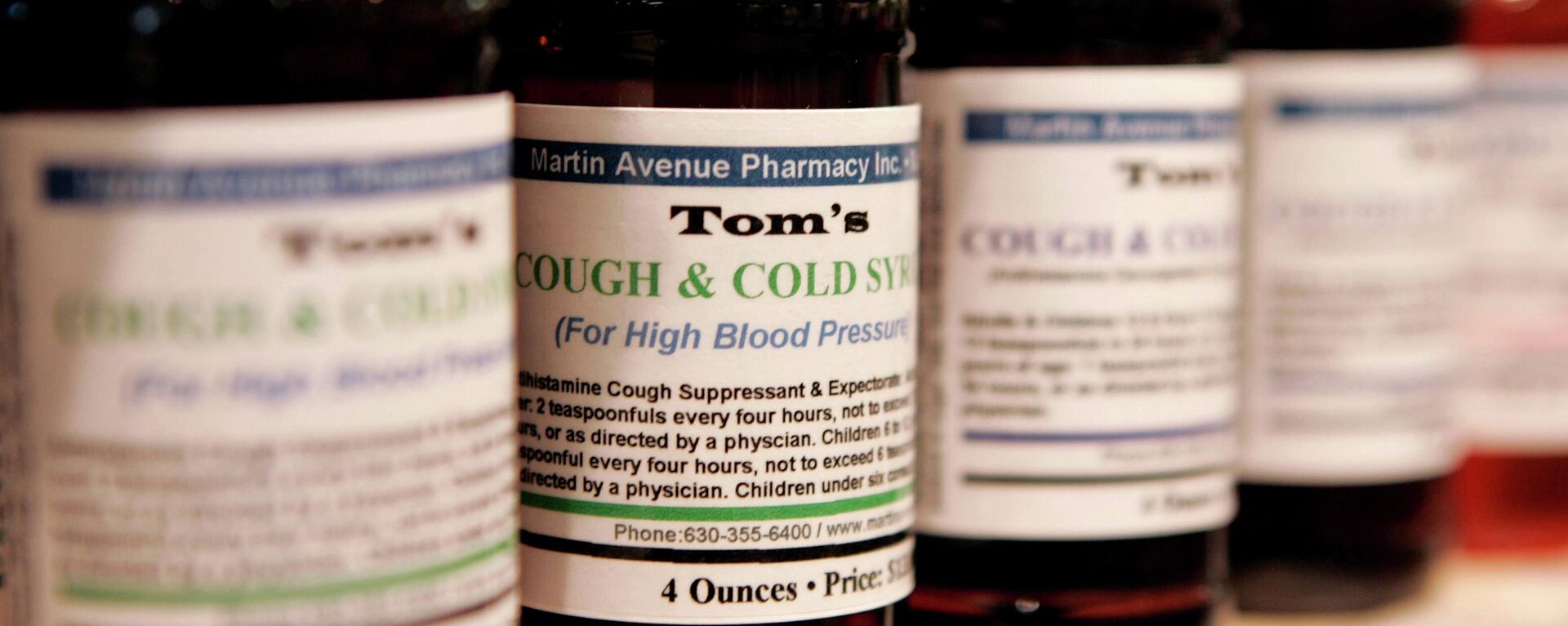
Indian health authorities are conducting a nationwide inspection of drug manufacturing facilities in order “to ensure safety, efficacy and quality of the drugs available in the country”.
2022-12-27T16:03+0000
2022-12-27T16:03+0000
2022-12-27T16:03+0000
africa
west africa
gambia
india
drugs
https://cdn1.img.sputnikglobe.com/img/07e6/0c/10/1105522219_0:160:3073:1888_1920x0_80_0_0_d82511014e178c9c8fdee98e5054acad.jpg
Indian health authorities are conducting a nationwide inspection of drug manufacturing facilities in order “to ensure safety, efficacy and quality of the drugs available in the country,” India’s Ministry of Health announced on Tuesday.The ministry’s efforts, which are conducted in cooperation with India’s pharmaceuticals regulator, the Central Drugs Standard Control Organization (CDSCO), came in the wake of a controversy involving four Indian-made cough syrups believed to have had a deadly effect on children. Nearly 70 children were reported to have died in Gambia after allegedly taking the Indian oral liquid solutions.The ministry stressed that the aim of the measures taken is to ensure that factories comply with all regulatory standards for the pharmaceutical industry, including the requirements of Good Manufacturing Practices (GMP).In October, the Indian government suspended all drug production plants of Maiden Pharmaceuticals following a warning by the World Health Organization that the cough syrups produced by the Indian company are likely to have been polluted by “unacceptable” levels of possibly toxic chemicals that led to the death of at least 66 children in Gambia.Indian authorities later sent samples from the same batches of the syrups sent to Gambia to an Indian government-owned laboratory for examination. In the end, the government concluded that the drugs were “safe” and complied with Indian standards.In mid-December, Maiden Pharmaceuticals announced that it was planning to resume production of its four oral liquid solutions following the Indian government's conclusion that the drugs were safe.
https://sputnikglobe.com/20221012/gambian-police-claim-cough-syrup-brought-by-us-company-to-blame-for-deaths-of-at-least-66-children-1101753971.html
africa
west africa
gambia
Sputnik International
feedback@sputniknews.com
+74956456601
MIA „Rossiya Segodnya“
2022
Muhammad Nooh Osman
https://cdn1.img.sputnikglobe.com/img/07e4/08/0e/1080170965_2:0:2050:2048_100x100_80_0_0_1de8233c87df0979e7e74f61b6ffacad.jpg
Muhammad Nooh Osman
https://cdn1.img.sputnikglobe.com/img/07e4/08/0e/1080170965_2:0:2050:2048_100x100_80_0_0_1de8233c87df0979e7e74f61b6ffacad.jpg
News
en_EN
Sputnik International
feedback@sputniknews.com
+74956456601
MIA „Rossiya Segodnya“
Sputnik International
feedback@sputniknews.com
+74956456601
MIA „Rossiya Segodnya“
Muhammad Nooh Osman
https://cdn1.img.sputnikglobe.com/img/07e4/08/0e/1080170965_2:0:2050:2048_100x100_80_0_0_1de8233c87df0979e7e74f61b6ffacad.jpg
whom is to blame for gambia children deaths, which cough syrups kill, acute kidney injuries and 66 deaths among children
whom is to blame for gambia children deaths, which cough syrups kill, acute kidney injuries and 66 deaths among children
India Conducts Nationwide Inspection of Drug Production Facilities in Wake of Gambia Child Deaths
Muhammad Nooh Osman
Writer/Editor
In October, Indian authorities put a halt to the production of four cough syrups after the World Health Organization issued a warning against the drugs for being “potentially linked with acute kidney injuries and 66 deaths among children” in Gambia. Later, India concluded that the drugs sent to Gambia were safe and complied with Indian standards.
Indian health authorities are
conducting a nationwide inspection of drug manufacturing facilities in order “to ensure safety, efficacy and quality of the drugs available in the country,” India’s Ministry of Health announced on Tuesday.
The ministry’s efforts, which are conducted in cooperation with India’s pharmaceuticals regulator, the Central Drugs Standard Control Organization (CDSCO), came in the wake of a controversy involving four Indian-made cough syrups believed to have had a deadly effect on children. Nearly 70 children were reported to have died in Gambia after allegedly taking the Indian oral liquid solutions.
“Joint inspections are being conducted all over the country as per standard operating procedures,” the Indian Ministry of Health said. “This will ensure high standards of quality compliance with respect to drugs manufactured in the country.”
The ministry stressed that the aim of the measures taken is to ensure that factories comply with all regulatory standards for the pharmaceutical industry, including the requirements of Good Manufacturing Practices (GMP).
“An action plan for nationwide inspection of manufacturing units which are identified to be at the risk of manufacturing Not of Standard Quality (NSQ)/adulterated/spurious drugs was made prior to carrying out of inspections,” the statement read.
In October, the I
ndian government suspended all drug production plants of Maiden Pharmaceuticals following a warning by the World Health Organization that the cough syrups produced by the Indian company are likely to have been polluted by “unacceptable” levels of possibly toxic chemicals that led to the death of at least 66 children in Gambia.
12 October 2022, 14:18 GMT
Indian authorities later sent samples from the same batches of the syrups sent to Gambia to an Indian government-owned laboratory for examination. In the end, the government concluded that the drugs were “safe” and complied with Indian standards.
In mid-December, Maiden Pharmaceuticals announced that it was planning to resume production of its four oral liquid solutions following the Indian government's conclusion that the drugs were safe.




