https://sputnikglobe.com/20221216/indian-lab-to-resume-production-of-safe-syrups-linked-to-death-of-66-gambian-children-1105522101.html
Indian Lab to Resume Production of 'Safe' Syrups Linked to Death of Nearly 70 Gambian Children
Indian Lab to Resume Production of 'Safe' Syrups Linked to Death of Nearly 70 Gambian Children
Sputnik International
This article is about Maiden Pharmaceuticals Ltd., the company that produced the cough syrups believed to have been linked to the death of nearly 70 Gambian children, announcing on Friday that it is planning to resume the production of its four oral liquid solutions in the near future after health authorities in India concluded that the drugs sent to Gambia were safe and complied with the Indian standards.
2022-12-16T14:47+0000
2022-12-16T14:47+0000
2022-12-16T14:51+0000
africa
west africa
gambia
world health organization (who)
india
world
health
https://cdn1.img.sputnikglobe.com/img/07e6/0c/10/1105522219_0:160:3073:1888_1920x0_80_0_0_d82511014e178c9c8fdee98e5054acad.jpg
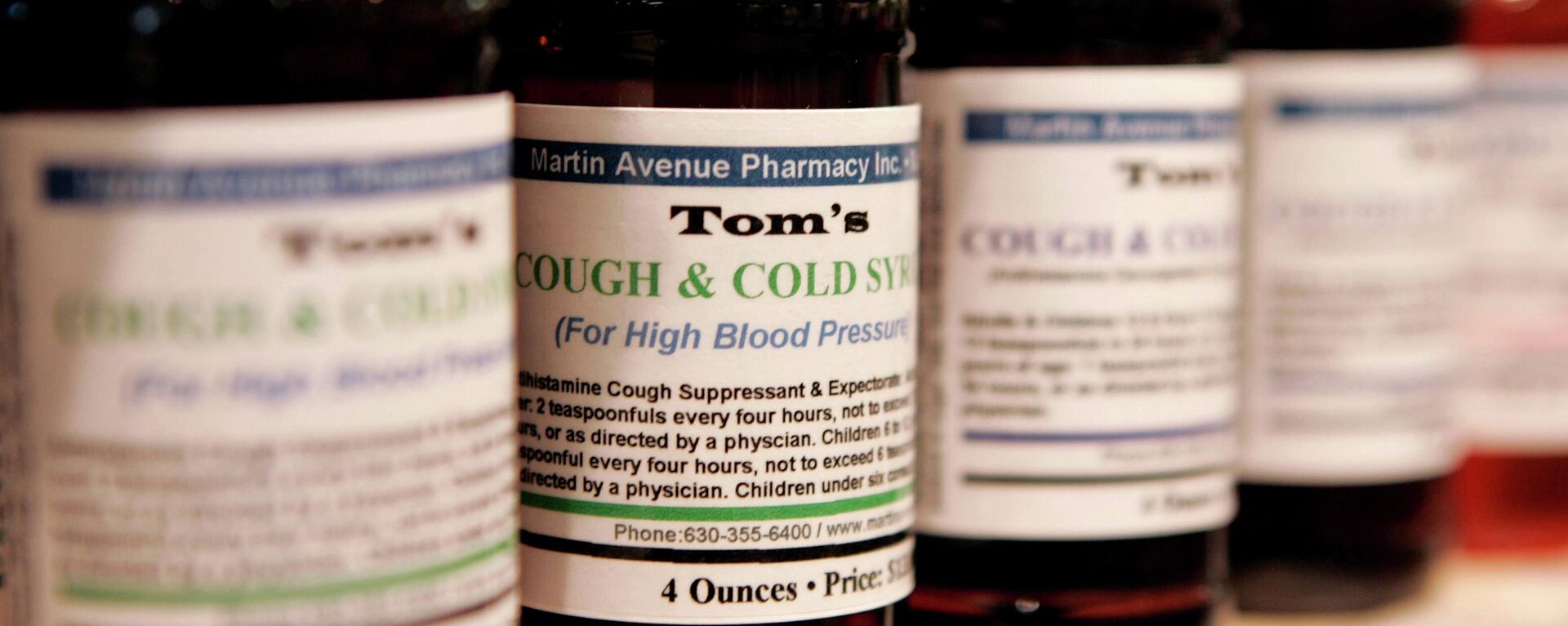
The company that produced the cough syrups believed to have been linked to the death of nearly 70 Gambian children announced on Friday that it is planning to resume production of its four oral liquid solutions. The move came after Indian health authorities concluded that the drugs sent to Gambia were safe and complied with Indian standards.Maiden Pharmaceuticals' main production plant was closed in October following a warning by the World Health Organization that the cough syrups are likely to have been exposed to “unacceptable” levels of possibly toxic chemicals found in the plant.The WHO’s warning came in the wake of reports about the death of at least 66 children in Gambia after taking cough syrups imported into the African country from India via a US-based pharmaceutical company. Later, Indian authorities sent samples from the same batches of the syrups sent to Gambia to a government-own laboratory for examination.However, a Health Ministry panel in India is currently reviewing the result of tests in order to take further action. It was reported earlier that India has officially told the WHO about the lab findings.For its part, the company claims to be innocent of shipping damaged syrups.In a letter to the World Health Organization earlier in the week, the Asian country’s Drugs Controller General Venugopal G Somani claimed that the WHO’s accusations regarding the Indian-made syrups had “adversely impacted the image of India's pharmaceutical products across the globe, and caused irreparable damage to the supply chain of pharmaceutical products.”In October, as part of its preliminary investigation, Gambian police said that four Indian-manufactured cough syrups that were brought to the African country by a US pharmaceutical firm were behind the death of 66 children. Following the reports, the WHO demanded India launch a probe into the matter, arguing that the syrups were potentially linked with acute kidney injuries responsible for the deaths.
https://sputnikglobe.com/20221012/gambian-police-claim-cough-syrup-brought-by-us-company-to-blame-for-deaths-of-at-least-66-children-1101753971.html
africa
west africa
gambia
world
Sputnik International
feedback@sputniknews.com
+74956456601
MIA „Rossiya Segodnya“
2022
Muhammad Nooh Osman
https://cdn1.img.sputnikglobe.com/img/07e4/08/0e/1080170965_2:0:2050:2048_100x100_80_0_0_1de8233c87df0979e7e74f61b6ffacad.jpg
Muhammad Nooh Osman
https://cdn1.img.sputnikglobe.com/img/07e4/08/0e/1080170965_2:0:2050:2048_100x100_80_0_0_1de8233c87df0979e7e74f61b6ffacad.jpg
News
en_EN
Sputnik International
feedback@sputniknews.com
+74956456601
MIA „Rossiya Segodnya“
Sputnik International
feedback@sputniknews.com
+74956456601
MIA „Rossiya Segodnya“
Muhammad Nooh Osman
https://cdn1.img.sputnikglobe.com/img/07e4/08/0e/1080170965_2:0:2050:2048_100x100_80_0_0_1de8233c87df0979e7e74f61b6ffacad.jpg
maiden pharmaceuticals ltd., gambian children, death of children in gambia, indian syrups, who india,
maiden pharmaceuticals ltd., gambian children, death of children in gambia, indian syrups, who india,
Indian Lab to Resume Production of 'Safe' Syrups Linked to Death of Nearly 70 Gambian Children
14:47 GMT 16.12.2022 (Updated: 14:51 GMT 16.12.2022) Muhammad Nooh Osman
Writer/Editor
The World Health Organization issued a warning against four Indian-made cough syrups in October for being “potentially linked with acute kidney injuries and 66 deaths among children” in Gambia. Later, Indian authorities put a halt on the production of the oral liquid solutions and sent samples of them to a federal laboratory for checks.
The company that produced the cough syrups believed to have been linked to the death of nearly 70 Gambian children announced on Friday that it is planning to resume production of its four oral liquid solutions.
The move came after Indian health authorities concluded that the
drugs sent to Gambia were safe and complied with Indian standards.
Maiden Pharmaceuticals' main production plant was closed in October following a warning by the World Health Organization that the cough syrups are likely to have been exposed to “unacceptable” levels of possibly toxic chemicals found in the plant.
The WHO’s warning came in the wake of reports about the death of at least 66 children in Gambia after taking cough syrups imported into the African country from India via a US-based pharmaceutical company. Later, Indian authorities
sent samples from the same batches of the syrups sent to Gambia to a government-own laboratory for examination.
However, a Health Ministry panel in India is currently reviewing the result of tests in order to take further action. It was reported earlier that India has officially told the WHO about the lab findings.
For its part, the company claims to be innocent of shipping damaged syrups.
“I have full faith in Indian regulatory and judiciary processes. I have not done anything wrong,” Maiden Managing Director Naresh Kumar Goyal said, as cited by the media. “We will now try to request the authorities to reopen the factory. But I don't know when that will happen. We are still waiting.”
In a letter to the World Health Organization earlier in the week, the Asian country’s Drugs Controller General Venugopal G Somani claimed that the
WHO’s accusations regarding the Indian-made syrups had “adversely impacted the image of India's pharmaceutical products across the globe, and caused irreparable damage to the supply chain of pharmaceutical products.”
12 October 2022, 14:18 GMT
In October, as part of its preliminary investigation, Gambian police said that four Indian-manufactured cough syrups that were brought to the African country by a US pharmaceutical firm were behind the death of 66 children. Following the reports, the WHO demanded India launch a probe into the matter, arguing that the syrups were potentially linked with acute kidney injuries responsible for the deaths.




