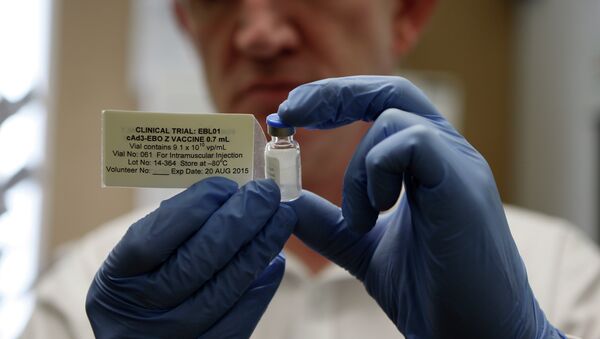
“Shipping the vaccine today [Friday] is a major achievement and shows that we remain on track with the accelerated development of our candidate Ebola vaccine,” Chairman of Global Vaccines at GSK Dr Moncef Slaoui, was quoted as saying in a statement posted on the company’s website.
We are shipping the first doses of our #Ebola vaccine candidate to Liberia for use in a clinical trial. Read more: http://t.co/ndZHK7ZN6N
— GSK (@GSK) 23 января 2015
Moncef added that the candidate Ebola vaccine, co-developed by the US National Institutes of Health, is still in the development phase and mass vaccinations would depend on whether the World Health Organization, and other regulators, were content with the protection it provided against the virus.
Based on results of the first clinical trials conducted on some 200 healthy individuals in the United Kingdom, the United States, Switzerland and Mali, the experimental vaccine shows an “acceptable safety profile,” according to the statement.
The current Ebola outbreak has killed over 8,000 people in the three most-affected countries of Liberia, Sierra Leone and Guinea, and over 20,000 probable, suspected and confirmed cases have been reported, according to the World Health Organization.
Meanwhile, the weekly numbers of new Ebola cases reported in all the three countries has declined to its lowest level in recent months.